 St. Luke’s Episcopal Hospital, home of the Texas Heart® Institute, is among the nation’s first cardiovascular centers to offer the Thoratec HeartMate® SNAP-VE left ventricular assist system (LVAS) as a permanent implant – called destination therapy – for end-stage congestive heart failure patients who do not qualify for heart transplantation due to age or other health circumstances.
St. Luke’s Episcopal Hospital, home of the Texas Heart® Institute, is among the nation’s first cardiovascular centers to offer the Thoratec HeartMate® SNAP-VE left ventricular assist system (LVAS) as a permanent implant – called destination therapy – for end-stage congestive heart failure patients who do not qualify for heart transplantation due to age or other health circumstances.
St. Luke’s is able to offer this device because in November 2002, the U.S. Food and Drug Administration (FDA) approved the HeartMate for permanent use in patients too sick to receive a heart transplant. The FDA’s approval expanded the availability of HeartMate to thousands of Americans with the most severe form of heart failure.
Doctors at St. Luke’s and Texas Heart Institute believe that this is the first truly acceptable solution for long-term support of heart failure patients because it allows them to leave the hospital and return to active, productive lives. The HeartMate is too large for implantation in most women and some men, however. In addition, the FDA notes that implanting the device requires major surgery with the risk of “life-threatening side effects.”
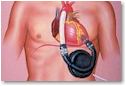
HeartMate is known as a left ventricular assist device because it helps the left ventricle – the heart's main pumping chamber – circulate blood throughout the body. The device consists of a pump that is implanted in the abdomen, which has an electrical cable and air vent that connects to external battery packs. The packs are worn on the shoulders and electronic controls are worn on the belt.
More than four million Americans have congestive heart failure, a chronic condition in which the heart loses its ability to pump blood efficiently. The disease causes fatigue and shortness of breath as fluid accumulates in the lungs and tissues. Leading causes of heart failure are damage to heart muscle from coronary artery disease or high blood pressure.
The FDA approved the HeartMate for destination therapy based on results of the landmark REMATCH clinical trial that demonstrated the device nearly doubled and tripled survival at one and two years respectively, and improved quality of life in this patient group. Physicians of St. Luke’s and the Texas Heart Institute have implanted 178 HeartMate devices since 1986.
St. Luke’s, home of the Texas Heart Institute, originated the laboratory and clinical research of the device, and implanted the first 20 HeartMates in the world. O.H. “Bud” Frazier, M.D., and his team initiated the research which led to the REMATCH study and have implanted more of the devices than any other heart center worldwide.
If you suffer from congestive heart failure and want to know if you may be a candidate for the HeartMate as a permanent implant, please e-mail us. We will reply to your e-mail within five working days.